Plant Guard Cells can Count Environmental Stimuli
10/21/2024Plants adapt their water consumption to environmental conditions by counting and calculating environmental stimuli with their guard cells. Plant researchers from Würzburg report this in ‘Current Biology’.
Plants control their water consumption via adjustable pores (stomata), which are formed from pairs of guard cells. They open their stomata when there is a sufficient water supply and enough light for carbon dioxide fixation through photosynthesis. In the dark and in the absence of water, however, they initiate the closing of the pores.
SLAC/SLAH-type anion channels in the guard cells are of central importance for the regulation of the stomata. This has been shown by the group of Professor Rainer Hedrich, biophysicist at Julius-Maximilians-Universität (JMU) Würzburg in Bavaria, Germany.
The anion channels are activated by calcium signals, which in turn are caused by environmental stimuli, such as a lack of water and nutrients, soil salinisation or infestation by pathogens. These calcium signals occur in different forms depending on the stimulus. Scientists therefore also refer to them as calcium signatures. A frequently occurring signature is the so-called calcium transient, a rapid, temporary increase in the calcium concentration in the cells.
Calcium Transient Follows All-or-nothing law
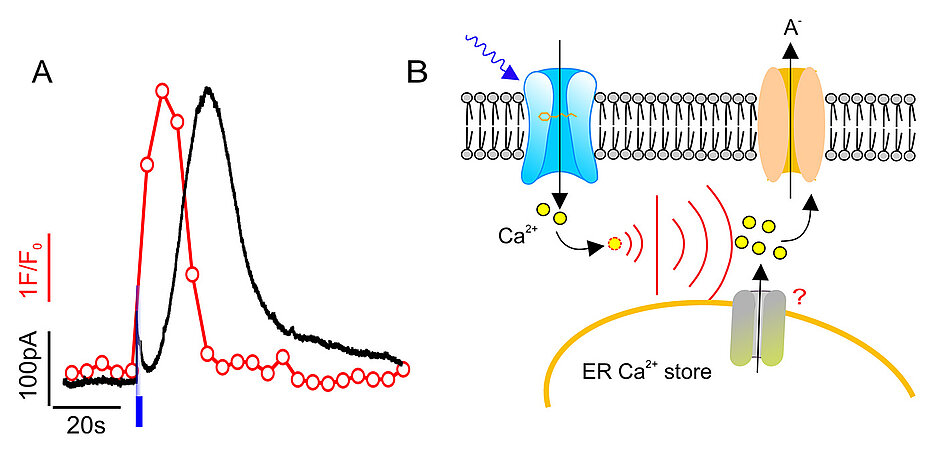
How much information is contained in a calcium transient? To answer this question, Hedrich's team has now used an optogenetic method with novel model plants that have been equipped with light-activated calcium channels: Light pulses can be used to generate calcium signals in the guard cells of these plants and analyse the cellular response.
‘We were quite surprised that light pulses of 0.1, one and ten seconds duration generated almost identical calcium transients,’ says Shouguang Huang, the first author of the paper published in the journal Current Biology: In the guard cells, the calcium concentration increased for 30 seconds after the light stimuli, only to subside again after a further 30 seconds.
‘We hypothesised that this all-or-nothing phenomenon occurs because the amount of calcium flowing in from the outside releases further calcium from stores inside the cell, which optimally amplifies the signal,’ explains Rainer Hedrich. The Würzburg plant scientists were right: when they inhibited calcium storage in the endoplasmic reticulum of the cell, the calcium transient and the subsequent reaction failed to materialise.
Anion Current Follows the Calcium Signal With a Time Delay
‘We were amazed a second time when, in addition to the calcium signal, we also observed the subsequent reaction in the guard cells, the swelling of the anion current,’ explains Shouguang Huang. As with the calcium transients, light pulses of different lengths triggered anion currents of similar shape and strength. The currents followed the calcium signal with a time delay: they only swelled after the calcium concentration in the cytosol had exceeded a threshold.
After the calcium transient ceased, however, the anion current could still be measured for a further 30 seconds. This lagging of the electrical signal is related to the biology of the enzymes that process the calcium signal and switch the anion channels on or off accordingly, explains Rainer Hedrich. This made it clear that the calcium influx lasting 0.1 seconds is amplified in the cell in such a way that a subsequent reaction lasting more than a hundred times longer is set in motion.
Guard Cells Can Count to Six
How many calcium transients are necessary for plants to close their stomata? To answer this question, the research team exposed guard cells to a 0.1 second light pulse every half a minute and observed the stomata. After the first pulse, the pore width decreased by 10 per cent, after three stimuli by 30 per cent, after six stimuli by 80 per cent and after 12 or more stimuli by 100 per cent.
‘This tells us that guard cells can resolve six consecutive calcium transients and convert them into stomatal movement. The guard cells can therefore count to six,’ says Rainer Hedrich. ‘When we doubled the stimulation frequency, stoma closure was not accelerated. When we halved it, stoma movement was delayed.’
On and On – the Next Research Questions
How will this research continue? ‘At the moment, we are looking for the step in the stimulus-response chain that depends on the frequency of the calcium transient and determines the speed. We are also interested in how guard cells decode the calcium signals and convert them into a number-dependent enzymatic-mediated activation of their anion channels,’ says the JMU biophysicist. In addition, the question of how long the guard cells remember the respective calcium signals must be clarified.
Publication
Guard cells count the number of unitary cytosolic Ca2+ signals to regulate stomatal dynamics. Shouguang Huang, M. Rob G. Roelfsema, Matthew Gilliham, Alistair M. Hetherington, Rainer Hedrich. Current Biology, 21 October 2024, https://doi.org/10.1016/j.cub.2024.07.086
Contact
Prof. Dr Rainer Hedrich, Chair of Botany I (Molecular Plant Physiology and Biophysics), University of Würzburg, T +49 931 31-86100, hedrich@botanik.uni-wuerzburg.de






