New Findings About Anti-Malaria Drug
01/30/2019Researchers at the Rudolf Virchow Center of the University of Würzburg have unveiled the molecular effectiveness of artemisinins. The findings could lead to drugs for diseases such as Alzheimer's, schizophrenia and epilepsy.
Artemisinin is derived from the leaves and flowers of the annual mugwort (Artemisia annua) and has been used in traditional Chinese medicine for centuries. The effectiveness was investigated by the Chinese researcher Tu Youyou. Her research was 2015 rewarded with the Nobel Prize. Artemisinin and its semi-synthetic derivatives – collectively known as artemisinins – are used to treat the tropical infectious disease malaria. In addition, these molecules also influence multiple cellular processes in humans. For example, artemisinins are able to activate the immune system against several types of cancer or to regulate the differentiation of pancreatic Ta cells, which could potentially be useful in the therapy of diabetes.
Molecular Mechanisms so far unknown
"Although this clinically-approved drug class is well established and has been used in some extent for centuries, it was unclear which molecular mechanisms underlie the corresponding cellular activities, such as target protein rfhumecognition and modulation," explains Dr. Vikram Kasaragod. The postdoctoral fellow in the research group of Professor Hermann Schindelin at the Rudolf Virchow Center is the first author of this article and ensures with this research work a significant gain in knowledge. The study was published in the journal Neuron.
Comprehensive model for the regulation of inhibitory neurotransmission developed
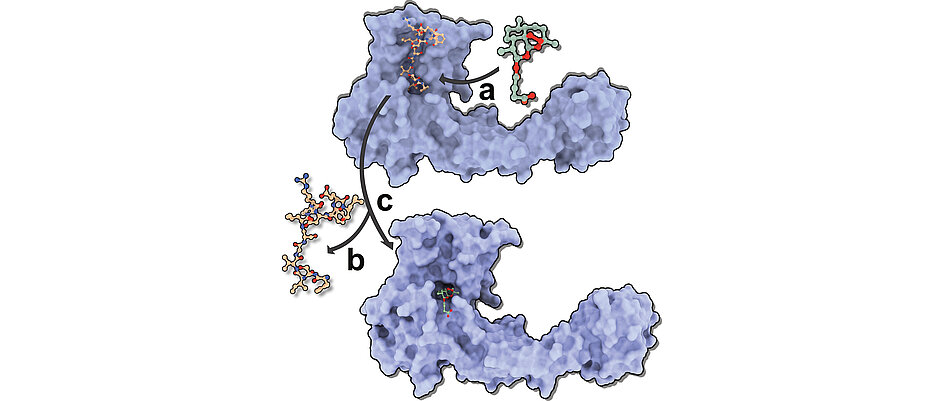
The structural biologist was the first to solve the crystal structures of two different artemisinin derivatives – artesunate and artemether – in a complex with gephyrin. By binding to inhibitory glycine and GABAA receptors, gephyrin acts as a central scaffold protein of inhibitory postsynapses in the mammalian central nervous system. Gephyrin has only recently been identified as an artemisinin target protein. The results clearly demonstrate how artemisinins target the universal receptor binding pocket in gephyrin and compete with the inhibitory neurotransmitter receptors for an overlapping binding site. These new findings could thus also serve as an effective tool to understand the physiology of the human brain.
According to Kasaragod, the crystal structures form, together with biochemical, electrophysiological and in vivo data, a comprehensive model of the regulation of inhibitory neurotransmission by artemisinine. According to him, this model clearly describes the interactions between proteins and drugs.
Important step for the development of drugs
"Our data not only provide a solid foundation for understanding how artemisinins are recognized by a target molecule, but will also help researchers to develop and optimize these agents into highly specific modulators of gephyrin. These modulators may play an important role in the treatment of neurological diseases such as Alzheimer's disease, schizophrenia and epilepsy," says Schindelin, the lead investigator.
The data published in Neuron are the result of an interdisciplinary collaboration with other groups at the University of Würzburg, the University Medical Center in Hamburg and the University of Copenhagen.
Publication
Vikram Babu Kasaragod, Torben Johann Hausrat, Natascha Schaefer, Maximilian Kuhn, Nikolaj Riis Christensen, Ingrid Tessmer, Hans Michael Maric, Kenneth Lindegaard Madsen, Christoph Sotriffer, Carmen Villmann, Matthias Kneussel and Hermann Schindelin: Elucidating the Molecular Basis for Inhibitory Neurotransmission Regulation by Artemisinins. Neuron (2019) https://doi.org/10.1016/j.neurin.2019.01.001
Contact
Dr. Vikram Kasaragod, Schindelin Group, Rudolf Virchow Center, vikram.kasaragod@virchow.uni-wuerzburg.de
Prof. Dr. Hermann Schindelin, Rudolf Virchow Center, T. +49 931 31-80382, hermann.schindelin@virchow.uni-wuerzburg.de
Dr. Daniela Diefenbacher, Press Office, Rudolf Virchow Center, T. +49 931 31-88631, daniela.diefenbacher@uni-wuerzburg.de