Kidney tumor: Genetic trigger discovered
06/18/2018Scientists at the University of Würzburg have identified new molecular biomarkers for rare kidney tumors in small children. These may be targets for new therapies.
It is malignant and can occur in the first months of infants' lives or even before birth: congenital mesoblastic nephroma (CMN). Fortunately, the kidney tumor is very rare and can often be cured with surgery. However, there are no other specific treatment options - also because of the as yet unclarified causes of this tumor.
An enzyme becomes hyperactive
Three subtypes of this kidney tumor are known. An international team of researchers has now identified a genetic driver for one of these subtypes. Jenny Wegert and Professor Manfred Gessler from the Department of Developmental Biochemistry at the Biozentrum of Julius-Maximilians-University Würzburg (JMU) were responsible for this breakthrough, together with Sam Behjati and Grace Collord from the Wellcome Sanger Centre (Cambridge) and Christian Vokuhl from the University of Kiel. The results of their work are published in the current issue of the journal Nature Communications.
"Three subgroups can be defined in congenital mesoblastic nephroma in tissue sections: classical, cellular and mixed nephromas," explains Manfred Gessler. A characteristic change in the chromosomes has long been known to trigger cellular CMN. In this case, two genes fuse with each other, which leads to the overshooting activity of an enzyme. No typical genetic alterations were known for the classical variant of CMN, however.
Novel mutation detected
This has now changed: "By genome analysis of tumor and blood samples from patients, we were able to detect a novel mutation of the receptor for the epidermal growth factor, EGFR, in over 70 percent of classical CMN," Wegert describes the central result of the study. The scientists discovered a doubling of the enzymatically active kinase region in the genome of the affected cells. As a result, the EGF receptor becomes overactive and the tumor cells are permanently stimulated to grow. Further investigations have shown that in the majority of cases the mixed variant of the tumor also has such an EGFR mutation.
In both the classical and cellular variants of CMN, the genetic alterations trigger one of the most important signalling pathways for the activation of cell growth: the so-called MAP kinase cascade. Among other things, they switch on the BRAF kinase present in the cell. "Interestingly, the gene for this protein was also mutated in some of the tumors in which neither the mutation responsible for the classical nor the cellular variant could be detected," explained Gessler.
In these cases, the affected gene lost a region responsible for inhibiting BRAF kinase activity, with the result that the protein is permanently active and the MAP kinase cascade remains switched on.
Parallels to tumors in adults
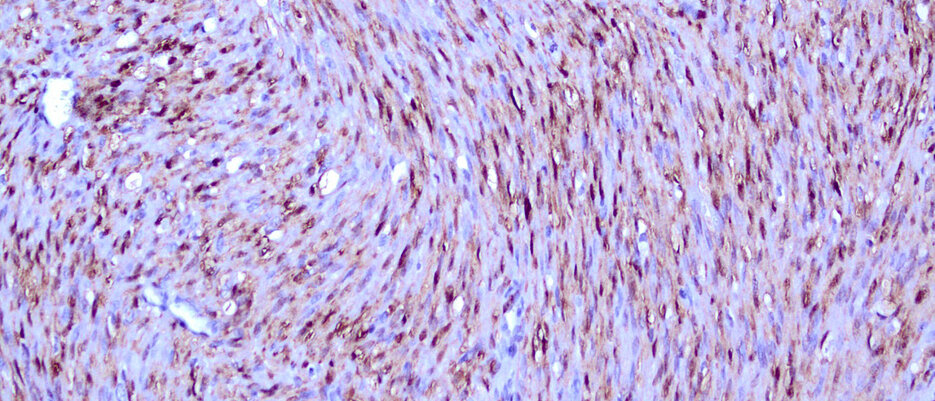
The research team has thus succeeded in almost all cases in attributing this rare tumor of infancy to the activation of a central signalling pathway, which also plays an important role in many adult tumors, which Professor Andreas Rosenwald from the Institute of Pathology at the JMU was able to substantiate with corresponding staining of tumor section.
Especially for CMN patients who cannot be adequately treated surgically, these findings may now provide new treatment approaches by transferring proven principles of adult oncology to the therapy of congenital mesoblastic nephroma therapy.
Recurrent intragenic rearrangements of EGFR and BRAF in soft tissue tumors of infants. Nature Communications, doi: 10.1038/s41467-018-04650-6, https://www.nature.com/articles/s41467-018-04650-6 .
University of Würzburg
Home to just under 29,000 students, Julius-Maximilians-Universität Würzburg (JMU) is one of the largest universities in Germany. True to its motto ‘Science for Society’, JMU is committed to advancing research in fields that are relevant for the future, focussing on eight pillars: Life Sciences / Health Sciences / Molecular Chemistry and Materials / Quantum Phenomena in New Materials / Digital Society / Cultural Heritage / Norms and Behavior / Global Changes.
The Wellcome Sanger Institute
The Wellcome Sanger Institute is one of the world's leading genome centres. Through its ability to conduct research at scale, it is able to engage in bold and long-term exploratory projects that are designed to influence and empower medical science globally. Institute research findings, generated through its own research programmes and through its leading role in international consortia, are being used to develop new diagnostics and treatments for human disease. To celebrate its 25th year in 2018, the Institute is sequencing 25 new genomes of species in the UK. Find out more at www.sanger.ac.uk.
Contact
Prof. Dr. Manfred Gessler, Chair of Developmental Biochemistry, T: +49 931 31 84159 / 84160 (Secr.), gessler@biozentrum.uni-wuerzburg.de






