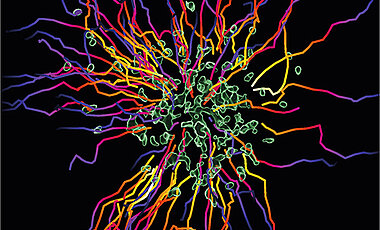
Start-stop System of Hunting Immune Cells
06/18/2021How immune cells coordinate their swarming behaviour to effectively eliminate pathogens: A publication in "Science" presents new findings.
The body is well protected against invading pathogens by barriers such as the skin. But if you injure yourself and break your skin, pathogens can easily enter your body through the wound and cause severe infections. If this occurs, the innate immune system takes over the first rapid defense with an effective arsenal of cellular weapons infiltrating the wounded tissue in large numbers.
As one of the first cell types on the spot, neutrophil granulocytes are recruited within few hours from the bloodstream to the infection site to eliminate potential microbial invaders.
Neutrophils are highly abundant cells that make up about 50-70% of white blood cells in the human body. It is estimated that 100 billion neutrophils are produced from stem cells in the bone marrow in an adult every day.
Swarming against infections
“Neutrophils are very efficient in hunting and killing bacteria,” says Tim Lämmermann. The group leader at the MPI of Immunobiology and Epigenetics in Freiburg studies this important cell type.
“These cells patrol almost all corners of our body, and they are very efficient in sensing anything potentially harmful in our body. Once individual neutrophils detect damaged cells or invading microbes in the tissue, they start secreting attractive signals that act through cell-surface receptors on neighboring neutrophils to recruit more and more cells.”
By using this intercellular communication, neutrophils can act together as a cell collective and coordinate effectively their clearance for pathogens as a swarm.
A fine line between host protection and tissue destruction
However, this form of beneficial inflammation can also overshoot and lead to massive tissue damage. If the intensity or the duration of the response becomes dysregulated, the same mechanisms that serve to eliminate invading pathogens can also cause collateral damage to healthy tissues. For example, the substances that neutrophils release to kill invading pathogens also erode the meshwork of proteins and sugars, which provides structural support to tissues.
“In this study, we started with the question what stops the swarming response to avoid uncontrolled neutrophil accumulation and prevent excessive inflammation, which can contribute to degenerative diseases such as cancer, diabetes, and autoimmune diseases,” says Tim Lämmermann.
In former studies, he and his team already discovered the molecular mechanisms initiating the collective-like swarming behavior. However, the processes that bring this response to an end have remained unknown.
Neutrophil swarming is still a relatively novel topic in the inflammation and infection research fields, and the underlying mechanisms are just beginning to be investigated. The newest study by the lab of Tim Lämmermann now reveals how neutrophils self-limit their swarming activity in bacteria-infected tissues and thus balance search versus destroy phases for efficient pathogen elimination.
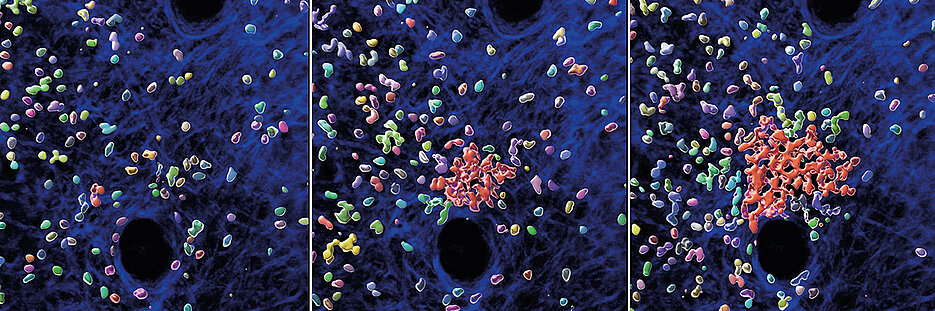
By using specialized microscopy for the real-time visualization of immune cell dynamics in living mouse tissues, the researchers demonstrate that swarming neutrophils become insensitive to their own secreted signals that initiated the swarm in the first place.
“We identified a molecular break in neutrophils that stops their movement, once they sense high concentrations of accumulating swarm attractants in large neutrophil clusters” says Tim Lämmermann.
“This was surprising as the prevailing view suggested that external signals released from the tissue environment or directly from pathogens are critical for stopping neutrophil activity in the resolution phase of an inflammation,” comments Wolfgang Kastenmüller, collaborating scientist of the Max Planck Research Group Systems Immunology at Julius-Maximilians-Universität (JMU) Würzburg in Bavaria, Germany.
An internal start-stop system for optimal bacterial clearance
In light of the discovered start-stop system in neutrophils, the researchers re-evaluated current views on how neutrophils navigate in tissues to eliminate bacteria efficiently.
In experiments with neutrophils lacking the stop mechanism, the team observed immune cells excessively swarming and scanning large areas of bacteria-infected tissue, which contrasted the behaviour of cells with functioning start-stop system.
However, this amplified swarming and scanning did not make these cells better pathogen-killers.
“Strikingly, we made the opposite finding. It is not beneficial when neutrophils move around too fast and run around like crazy. Instead, it appears more advantageous for them to stop and enjoy together a nice meal of bacteria – this is more efficient to contain bacterial growth in tissues,” explains Tim Lämmermann.
With these results, the team paves the way for a better understanding of neutrophil biology, which is essential for immune host defense against bacteria and could inform therapeutic approaches in the future. Moreover, the swarming behavior and underlying mechanisms could also inform other categories of collective behavior and self-organization in cells and insects.
Publication
Neutrophils self-limit swarming to contain bacterial growth in vivo. Kienle K, Glaser KM, Eickhoff S, Mihlan, M, Knöpper K, Réategui E, Epple MW, Gunzer M, Baumeister R, Tarrant TK, Germain RN, Irimia D, Kastenmüller, W, Lämmermann T (2021). In: Science 372, DOI: 10.1126/science.abe7729