Adrenocortical carcinoma: No mitotane for low risk of recurrence
08/25/2023After the complete tumour resection, not all patients with an adrenocortical carcinoma require the previous standard therapy Mitotane. Professors Martin Fassnacht and Massimo Terzolo show this in a clinical trial.
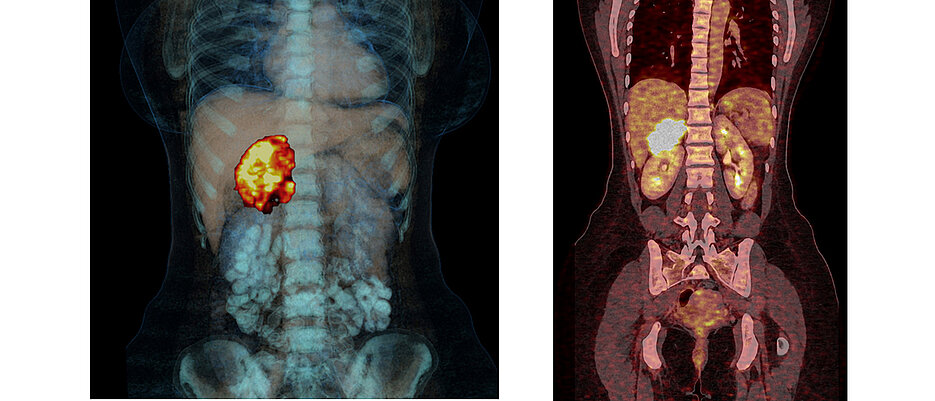
In 2017, the teams of Massimo Terzolo and Martin Fassnacht published a study in the New England Journal of Medicine that provided evidence for the efficacy of Mitotane in the prevention of recurrence in adrenocortical carcinoma. This study established the drug worldwide as a standard therapy for relapse prophylaxis after surgical removal of the tumour, regardless of risk factors that were still unknown at the time.
Mitotane inhibits cell division in the adrenal cortex and thus counteracts tumour growth. The risk of the disease coming back after surgery was three times higher in the control study group that did not receive Mitotane than in the Mitotane group. And the risk of dying from the disease was almost halved by the therapy. The Italia-German team had set new standards in the treatment of the very rare but extremely aggressive tumour.
Publication in The Lancet Diabetes & Endocrinology
"Our findings from 2007 still apply, but only to patients with normal or high risk of recurrence," explains Professor Martin Fassnacht, Chair of the Department of Endocrinology and Diabetology at Würzburg University Hospital.
In a new clinical study published in August 2023 in the journal The Lancet Diabetes & Endocrinology, he and Massimo Terzolo and other collaborators found that the adjuvant treatment with Mitotane is not necessary if the patients fulfil three factors.
First: The operation was complete, so-called R0 resection. Second: The tumour stage was low and there had not yet been any spread. Third: The cell proliferation marker Ki-67 is below 10 percent. So if the risk of recurrence is low.
No improvement with low risk of recurrence
ADIUVO is the first ever randomised trial worldwide of adjuvant treatment for adrenocortical carcinoma. A total of 91 patients in 23 centres in seven countries were randomised to either receive oral Mitotane for two years or to be monitored "only" by imaging and laboratory controls after surgical removal of their adrenocortical carcinoma and low to intermediate risk of recurrence (R0 resection, stage I-III, Ki67 ≤10%).
The efficacy of Mitotane versus surveillance only was assessed by recurrence-free survival (RFS). The 5-year RFS rate was 79% in the Mitotane group and 75% in the surveillance group. The 5-year overall survival rate was not statistically significantly different.
However, all study participants who received Mitotane experienced adverse events, and eight people discontinued treatment. Mitotane treatment can be associated with nausea, diarrhoea, dizziness and even speech problems. Patients who did not want to be randomised were followed up in a prospective follow-up study. These were 95 people, of whom 42 were followed up with mitotane and 53 without. In this parallel study, the result of the randomised study was confirmed.
A first step towards personalised medicine in this rare disease
Martin Fassnacht sums up: "Concomitant therapy with Mitotane is not indicated in patients with low-grade, localised adrenocortical carcinoma in which the tumour has not yet metastasised and could be completely removed, as their prognoses are relatively good and treatment with Mitotane does not show a statistically significant improvement in the relapse rate, but is associated with side effects."
In other words, "our study is a first step towards personalised medicine in this rare disease. It shows that it is possible, with a simple and widely available prognostic scheme based on tumour stage, resection status and Ki-67 assessment, to identify a subgroup of patients whose prognosis is much better than expected and for whom active surveillance is the most appropriate approach."
Würzburg endocrinology has decisively shaped international therapy standards
Endocrinology at Würzburg University Hospital is considered an international reference centre for the diagnosis, treatment and research of adrenocortical carcinoma and is currently the largest centre worldwide.
The usually highly malignant tumour of this endocrine gland, which sit in pairs on the kidneys, has been the focus of Würzburg endocrinology for more than 20 years. The European Adrenal Tumour Network ENSAT and the German Adrenal Carcinoma Study Group are coordinated from here.
"With our numerous basic research, translational and clinical studies, we in Würzburg, together with a large interdisciplinary team, have made a significant contribution to improving the worldwide diagnosis and treatment of patients with adrenal carcinoma," Martin Fassnacht sums it up.
In Germany 80 to 120 new cases every year
Even the diagnosis is difficult, because adrenal carcinoma is very rare and initially causes no symptoms. Therefore, it is often only discovered at an advanced stage. In Germany, it is estimated that there are about 80 to 120 new cases every year. Depending on the type of tumour, surgery can be performed, and in the advanced stage, chemotherapy or radiotherapy are also necessary.
Until now, it was common practice to treat all patients with additional medication after surgery, regardless of the stage of the tumour. Currently, Mitotane is the only approved drug for adrenal carcinoma. Clinical studies on other drugs and therapies are underway at the University Hospital of Würzburg.
Publication
Adjuvant mitotane versus surveillance in low-grade, localised adrenocortical carcinoma (ADIUVO): an international, multicentre, open-label, randomised, phase 3 trial and observational study. The Lancet Diabetes & Endocrinology, 21. August 2023, ISSN 2213-8587, https://doi.org/10.1016/S2213-8587(23)00193-6