Cervical Cells Have Their Own Immune Intelligence
10/07/2025Epithelial cells lining the cervix are not just passive barriers. As a recently published study shows, these cells have their own “immune intelligence” and can prepare defenses before an infection spreads.
Sexually transmitted infections are among the most common infections worldwide, affecting more than a billion people. They contribute to infertility and complications during pregnancy and increase the risk of various types of cancer. The mucous membrane of the female reproductive tract, especially in the cervix, plays an essential role in these processes. The question of how this tissue perceives and potentially defends against pathogens is therefore of crucial importance for global health.
A research team from Aarhus has now gained new insights into these processes. The scientists were able to show that epithelial cells in the cervix itself coordinate the immune response. “They are not passive walls, but active guardians of tissue health,” says Prof. Dr. Cindrilla Chumduri, study leader and lead author of the study now published in the journal Science Advances.
An Immunocompetent Tissue
According to the research team, this discovery changes the way we view the cervix: “It is not just a barrier, but an immunocompetent tissue that can coordinate complex defense mechanisms,” says Cindrilla Chumduri. The new findings thus offer a new approach to infection biology and have implications for a range of applications, such as:
• Mucosal vaccines targeting epithelial defenses
• Therapies to boost innate protection against both bacterial and viral sexually transmitted infections.
In addition, they provide an approach for better prevention of infection-driven cancers and infertility.
Cindrilla Chumduri is an infection and cancer biologist who has been researching the physiological processes in cervical tissue for many years, first as a group leader at the Max Planck Institute for Infection Biology, Berlin and Department of Microbiology at Julius Maximilian University of Würzburg (JMU), and now as a professor at the Department of Biological and Chemical Engineering at Aarhus University.
“Anatomically speaking, the cervix is a complicated structure,” says the scientist. The link between the uterine cavity and the vagina consists of the endocervix, which is adjacent to the uterus, and the ectocervix, which protrudes into the vagina. These are lined with different types of cells: while the endocervix has a columnar epithelium, the ectocervix has a multilayered squamous epithelium.
Two Regions, Two Defense Strategies
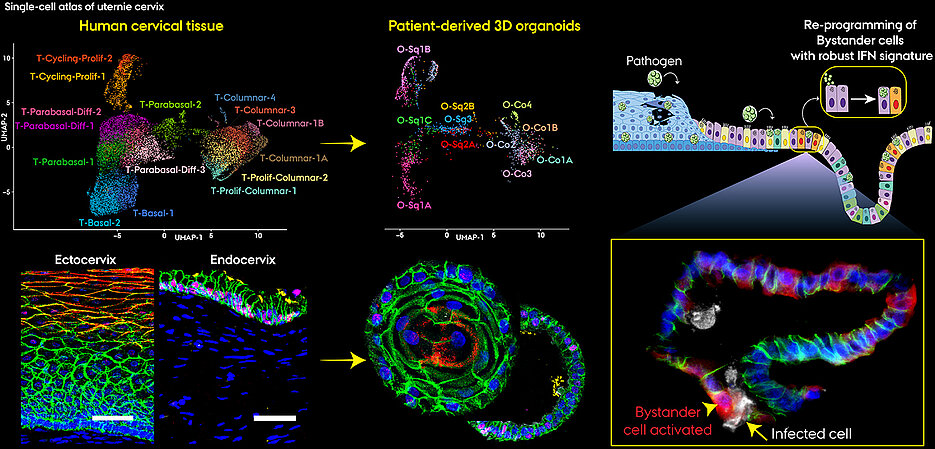
For its recently published study, the research team relied on 3D organoid models. With their three-dimensional tissue architecture and composition, these laboratory cultures resemble their natural counterparts and retain the functional properties of the original tissue. By benchmarking organoids against primary cervical tissue at single-cell resolution, the team demonstrated that these models faithfully reproduce in vivo epithelial subtypes and their defense programs. Such models are increasingly recognized as discovery platforms, and the work of the Chumduri Lab illustrates how organoid systems can drive new insights into infection and cancer biology.
Using a special technique called single-cell RNA sequencing, Chumduri and her team have mapped for the first time how thousands of individual epithelial cells respond to infection with Chlamydia trachomatis, the most common pathogen causing sexually transmitted diseases.
This revealed that:
• Ectocervical squamous cells focus on reinforcing the barrier.
• Endocervical columnar cells act as immune signalers, switching on interferon pathways, antimicrobial defenses, and antigen presentation, even when uninfected.
Subtypes with Specific Tasks
The team found that within each region, specialized epithelial subtypes carried out distinct tasks. In the ectocervix, some subtypes focused on regeneration and repair. In the endocervix, bystander cells, never directly infected, were the most immune-active.
“The bystander cells surprised us most,” says Dr. Pon Ganish Prakash, first author, who carried out the computational analysis of single-cell sequencing data. “They became the dominant defenders, amplifying immune signals without direct infection.”
Cellular Conversations
The team also decoded how epithelial subtypes talk to each other using chemical signals, revealing a hidden “conversation” that balances defense with repair.
“Working with these organoid models allowed us to recreate infection dynamics in a controlled and realistic way,” explains Dr. Naveen Kumar Nirchal. “We could see how certain epithelial subtypes act as hubs, sending signals that mobilize their neighbors.”
“Our findings show that epithelial heterogeneity is essential. Each subtype has its own job in protecting the cervix and preventing spread of infections to upper reproductive organs,” Dr. Rajendra Kumar Gurumurthy, senior scientist adds.
Original Publication
Single-cell atlas of cervical organoids uncovers epithelial immune heterogeneity and intercellular crosstalk during Chlamydia infection. Pon Ganish Prakash, Naveen Kumar Nirchal, Stefanie Köster, Christian Wentland, Jayabhuvaneshwari Dhanraj, Rajendra Kumar Gurumurthy, Cindrilla Chumduri. Science Advances, DOI: 10.1126/sciadv.ady1640
Contact
Prof. Dr. Cindrilla Chumduri, Medical Biotechnology Section, Department of Biological and Chemical Engineering, Aarhus University cindrilla.chumduri@bce.au.dk