P 3: V. Kozjak-P. / T. Rudel
Bacterial and host cell factors important for the invasion and dissemination of N. gonorrhoeae
State of the art
Neisseria gonorrhoeae is an obligate human pathogen that causes the sexually transmitted disease gonorrhea, with complications such as pelvic inflammatory disease, sterility, as well as the disseminated gonococcal infection. The number of new infections with N. gonorrhoeae continues to rise, and the emergence of broad-spectrum antibiotic resistance has recently afforded the bacteria ‘superbug’ status. In addition to cancer cell lines, human fallopian tubes in organ culture have been used as a model of choice when studying N. gonorrhoeae infection. The organ culture, however, has its limitations, since the previous medical history and endocrinological status of the donor introduce variables that can interfere with conclusions. Cell culture and especially tumor cell lines have serious drawbacks, as well, and are not suitable for addressing questions such as invasion of the underlying tissues or crossing of the endothelial barrier. To understand the transcytosis of N. gonorrhoeae and its dissemination into the blood stream, we are in need of better tissue models where these questions can be studied.
Previous Work
During infection, N. gonorrhoeae depends on type IV pili for the initial adhesion and Opa proteins and porin PorB that mediate invasion. In the case of the disseminated infection, the majority of the bacterial clinical isolates contain PorBIA variant of porin [1, 2]. The research of the Kozjak-Pavlovic group focused on PorBIA structure [2] and interaction with cell surface and mitochondria during infection [1, 3, 4, 5]. We have created several neisserial strains carrying point mutations in PorBIA, which impair its function in mediating cellular invasion [4]. The research further included creating a library of transposon-mediated mutants of N. gonorrhoeae, which is being tested using cervical carcinoma and conjunctiva cells as models, to discover uncharacterized bacterial pathogenicity factors responsible for the induction of cell death and invasion [6]. At the same time, we are using a library of cervical carcinoma cells with an shRNA-mediated downregulation of genes to search for host cell factors that are necessary for the initial adherence and invasion of N. gonorrhoeae.
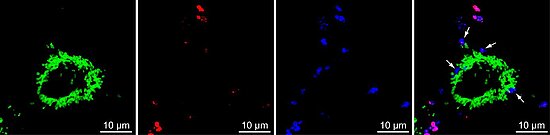
Figure: Invasive Neisseria gonorrhoeae in Chang cells. The green channel represents mitochondria-localized GFP, the red channel represents adherent N. gonorrhoeae and the blue channel represents adherent and invasive N. gonorrhoeae stained with Neisseria-specific antibody. In the overlay picture, invasive N. gonorrhoeae are marked with arrows.
Work Plan
At this point we are in need of better models that would enable us not only to look at the initial stages of the neisserial infection, but to follow the complete infection, including adhesion, transcytosis and transport to deeper tissue layers, as well as dissemination into the blood stream. We plan to set up simpler tissue models first, using immortalized cervical epithelial cells and fimbrial cells of human fallopian tubes on a collagen scaffold in a cell crown. We will perform this work in cooperation with the group of Heike Walles.
N. gonorrhoeae infection will be established to observe the adhesion, invasion and transcytosis of bacteria, as well as apoptosis induction and mitochondrial damage using fluorescent microscopy and transmission electron microscopy. We plan additionally to modulate oxygen levels and emulate shear stress and other environmental conditions, recruiting knowledge from the Schubert-Unkmeir group. Infecting the 3D tissue models with the available N. gonorrhoeae transposon library will help us to identify bacterial factors involved in an increased or reduced adhesion, invasion or tissue damage. The targets obtained from the screens where we look for the host cell factors important for neisserial invasion will be tested on the tissue model, as well.
The tissue model should be further developed and improved by adding a connective tissue layer below the epithelial cells and, eventually, introducing human microvascular endothelial cells on the basal side to simulate the endothelial barrier. At this stage, we can perform further experiments using transposon library to identify neisserial and host cell factors that contribute to the crossing of the endothelial barrier. To explore the role of PorBIA in this process, we will follow the adhesion and trancytosis efficiency of the point mutants of PorBIA we have already created. Using this improved tissue model, we will focus specifically on the bacterial crossing of the endothelial barrier and dissemination into the blood stream.
References
- Faulstich et al. (2014) Neutral sphingomyelinase 2 is a key factor for PorB-dependent invasion of Neisseria gonorrhoeae. Cell Microbiol. doi: 10.1111/cmi.12361. PubMed
- Zeth et al. (2013) Structure and function of the PorB porin from disseminating N. gonorhoeae, Biochem. J. 449:631-642. PubMed
- Rudel et al. (2010) Interactions between bacterial pathogens and mitochondrial cell death pathways. Nat. Rev. Microbiol. 8:693-705. PubMed
- Kozjak-Pavlovic et al. (2009) Bacterial porin disrupts mitochondrial membrane potential and sensitizes host cells to apoptosis. PLOS Path., 5:e1000629. PubMed
- Kozjak-Pavlovic et al. (2008) Import of bacterial pathogenicity factors into mitochondria. Curr. Opin. Microbiol., 11:9-14. PubMed
- Remmele et al. (2014) Transcriptional landscape and essential genes of Neisseria gonorrhoeae. Nucleic Acids Res. 42:10579-10595. PubMed






