Time-resolved fluorescence on extended time scales
1) Unraveling the hidden temporal range of fast b2-adrenergic receptor mobility and interactions
- in collaboration with Prof. Martin Lohse
G-protein-coupled receptors (GPCRs) are hypothesized to possess inter- and intramolecular mobility over a wide temporal range. Until now the temporal range has not been fully accessible due to the crucially limited temporal range of available methods. This in turn, may lead relevant dynamic constants to remain masked. We expand this dynamic range by combining fluorescent techniques using a spot confocal setup. We decipher mobility constants of β2-adrenergic receptor over a wide time range (nanosecond to second). Particularly, a translational mobility (10 µm²/s), one order of magnitude faster than membrane associated lateral mobility that explains membrane protein turnover and suggests a wider picture of the GPCR availability on the plasma membrane. And a so far elusive rotational mobility (1-200 µs) which depicts a previously overlooked dynamic component that, despite all complexity, behaves largely as predicted by the Saffman-Delbrück model. More interesting insights into the intramolecular dynamics of GPCRs upon ligand binding, we gain by Fluorescence Correlation Spectroscopy in combination with FRET in live cells. Stay tuned!
Publication: Unraveling the hidden temporal range of fast β2-adrenergic receptor mobility by time-resolved fluorescence. Balakrishnan A, Hemmen K, Choudhury S, Krohn JH, Jansen K, Friedrich M, Beliu G, Sauer M, Lohse MJ*, Heinze KG*. Commun Biol. 2022 Feb 28;5(1):176. doi: 10.1038/s42003-022-03106-4. *corresponding author
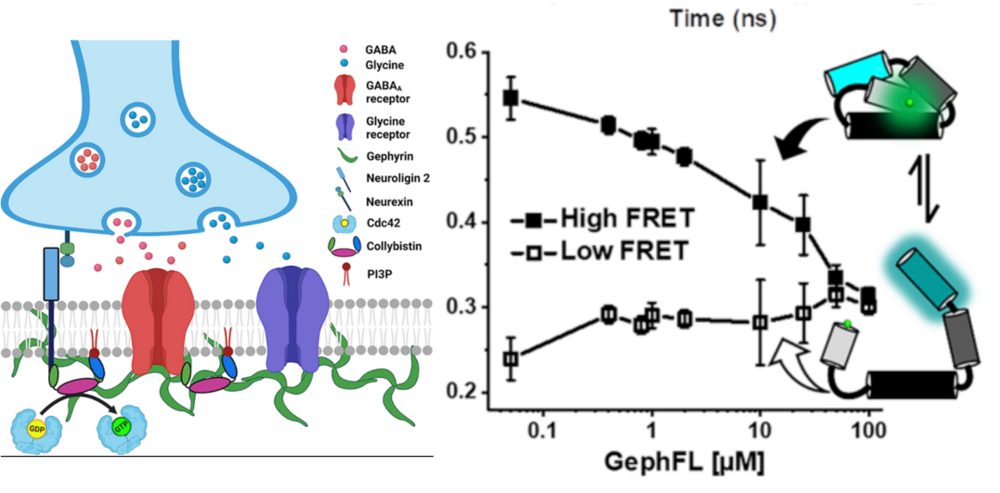
2) Conformational dynamics of gephyrin-mediated collybistin activation
- in collaboration with Prof. Hermann Schindelin
Efficient neuronal signaling depends on the proper assembly of the postsynaptic neurotransmitter machinery. The majority of inhibitory synapses feature γ-aminobutyric acid type A (GABAA) receptors. The function of these GABAergic synapses is controlled by the scaffolding protein gephyrin and collybistin, a Dbl family guanine nucleotide exchange factor and neuronal adaptor protein. Specifically, collybistin interacts with small GTPases, cell adhesion proteins, and phosphoinositides to recruit gephyrin and GABAA receptors to postsynaptic membrane specializations. Collybistin usually contains an N-terminal SH3 domain and exists in closed/inactive or open/active states. Here, we elucidate the molecular basis of the gephyrin-collybistin interaction with newly designed collybistin Förster resonance energy transfer (FRET) sensors. Using fluorescence lifetime-based FRET measurements, we deduce the affinity of the gephyrin-collybistin complex, thereby confirming that the C-terminal dimer-forming E domain binds collybistin, an interaction that does not require E domain dimerization. Simulations based on fluorescence lifetime and sensor distance distributions reveal at least a two-state equilibrium of the SH3 domain already in the free/unbound collybistin, thereby illustrating the accessible volume of the SH3 domain. Finally, our data provide strong evidence for a tightly regulated collybistin-gephyrin interplay, where, unexpectedly, switching of collybistin from closed/inactive to open/active states is efficiently triggered by gephyrin. Next, we will test our hypothesis in cells…stay tuned.
Publication:
- Deciphering the conformational dynamics of gephyrin-mediated collybistin activation. Imam N, Choudhury S, Hemmen K, Heinze KG, Schindelin H. / Biophys Rep (N Y). 2022 Sep 16;2(4):100079. doi: 10.1016/j.bpr.2022.100079. eCollection 2022 Dec 14.PMID: 36425671
- Differential modulation of collybistin conformational dynamics by the closely related GTPases Cdc42 and TC10. Imam N, Choudhury S, Heinze KG, Schindelin H. / Front Synaptic Neurosci. 2022 Aug 4;14:959875. doi: 10.3389/fnsyn.2022.959875. eCollection 2022.