Identification of a ferroptosis suppressor protein allows for new anticancer treatment approach
10/21/2019A team of researchers lead by Helmholtz Zentrum München and the University of Würzburg identified an enzyme as a novel and strong inhibitor of ferroptosis, the iron dependent form of cell death: ferroptosis suppressor protein-1, short FSP1. This protein is expressed in a variety of cancer cell lines and therefore represents an attractive drug target for cancer treatment. The results were published in the journal Nature.
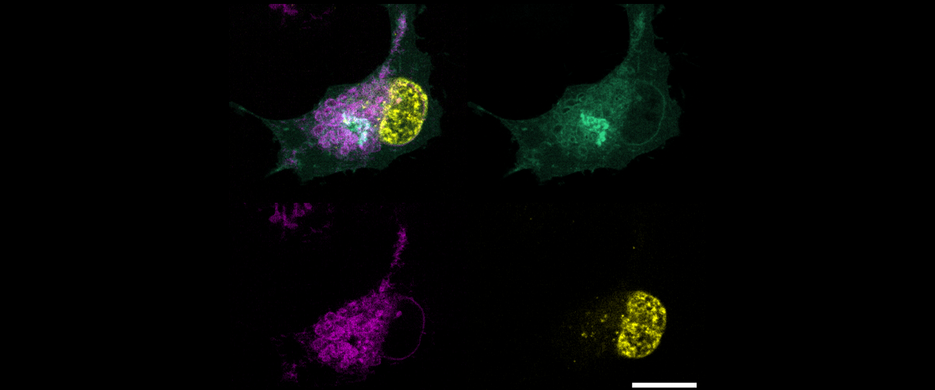
In fact, the first FSP1 inhibitor described in the researchers’ study published in Nature sensitizes tumor cells to ferroptosis, providing the rationale for the development of stratified combination therapies.
Development of drug resistance to chemotherapy represents a major clinical problem for the treatment of a variety of tumor entities. Metabolic alternations, epithelial to mesenchymal transition and immune evasion are some of the processes involved, which have been recently linked to an acquired sensitivity towards ferroptosis. So called “persister” cancer cells withstand conventional therapies and lead to tumor relapse. Interestingly, some persister cells are known to develop a strong dependency on glutathione peroxidase 4 (GPX4), the established regulator of ferroptosis. So far, GPX4 has been considered the sole enzymatic system in mammalian cells that can efficiently reduce and thereby detoxify peroxides in phospholipid bilayers by using the tripeptide glutathione, the most abundant natural antioxidant in mammalian cells.
FSP1 is a strong anti-ferroptotic gene expressed in most cancer cells
In order to unravel novel ways of using ferroptosis for cancer treatment researchers investigated the mystery of why certain cancer cells are resistant to ferroptosis despite expressing ACSL4 – a strong predictive marker for ferroptosis sensitivity (Doll et al Nat Chem Biol 2017). The team of researchers including Dr. Sebastian Doll and Dr. Marcus Conrad, both from the Institute of Developmental Genetics at Helmholtz Zentrum München, as well as Dr. Florencio Porto Freitas and Dr. José Pedro Friedmann Angeli from the University of Würzburg (Rudolf Virchow Center) performed a robust genetic suppressor screen using a cDNA library from a ferroptosis resistant breast cancer cell line. This screen led to the identification of FSP1, previously erroneously annotated as AIFM2 (Apoptosis Inducing Factor Mitochondria Associated 2), as a strong anti-ferroptotic gene.
FSP1 fully complements the loss of GPX4, the hitherto only known enzyme efficiently preventing lipid peroxidation and associated ferroptosis. Determining the biochemical function of FSP1, the researchers found that inhibition of lipid peroxidation depends on the presence of ubiquinone (also known as coenzyme Q10).
“The mechanism by which FSP1 protects cells from ferroptosis is unique and novel. FSP1 keeps ubiquinone in its reduced state and by this, ubiquinone protects membranes from radical driven lipid peroxidation. Ultimately, we found that this mechanism prevents the execution signal of ferroptosis,” explains Dr. Friedmann Angeli.
“In a next step, we screened approx. 10,000 drug-like compounds in a phenotypic cell-based screen and target-based validation assay and identified iFSP1 as the first FSP1 inhibitor,” says Dr. Bettina Proneth from the team at Helmholtz Zentrum München.
Dr. Sebastian Doll first author of the study further states: “Since we found that FSP1 is expressed in most cancer cell lines, novel FSP1 inhibitors have the potential to be further developed as anticancer drugs.”
Significant impulse for future ferroptosis-based cancer therapies
With this study, the team of researchers described an until now unknown mechanism of how to protect cells from lipid peroxidation induced ferroptotic cell death. Moreover, they showed that this system can provide strong resistance towards known ferroptosis inducing drugs. They further provided an explanation for the long-sought riddle why cells have large amounts of extra-mitochondrial ubiquinone.
“This study has widespread implications. It asks for the development of novel and target-based ferroptosis inducers, suggests the development of FSP1 as a biomarker for future ferroptosis-based therapies and seeks the re-evaluation of ubiquinone as a powerful anti-ferroptotic agent,” concludes Dr. Marcus Conrad.
Cancer therapy is often highly effective in the initial phase of treatment, but in many cases the patients’ tumor stops responding. As certain therapy-resistant tumors and those undergoing dedifferentiation evolve into a ferroptosis-sensitive state, pro-ferroptotic treatment paradigms based on inhibition of FSP1, GPX4 or a combination thereof ask to be translated into clinical applications in the future.
Munich in the center of international research
This research was funded by Helmholtz Zentrum München’s Development Grant for FSP1 inhibitors (2018) and the Deutsche Forschungsgemeinschaft. The study initiated at Helmholtz Zentrum München and developed in close collaboration with Rudolf-Virchow-Zentrum at Würzburg University, counted with important contributions from Cardiff University, University of Ottawa, Imperial College London and the University of California, Berkeley.
Publication
Sebastian Doll, Florencio Porto Freitas, Ron Shah, Maceler Aldrovandi, Milene Costa da Silva, Irina Ingold, Andrea Goya Grocin, Thamara Nishida Xavier da Silva, Elena Panzilius, Christina Scheel, André Mourão, Katalin Buday, Mami Sato, Jonas Wanninger, Thibaut Vignane, Vaishnavi Mohana, Markus Rehberg, Andrew Flatley, Aloys Schepers, Andreas Kurz, Daniel White, Markus Sauer, Michael Sattler, Edward William Tate, Werner Schmitz, Almut Schulze, Valerie O’Donnel, Bettina Proneth, Grzegorz M. Popowicz, Derek Pratt, José Pedro Friedmann Angeli, Marcus Conrad: FSP1 is a glutathione-independent ferroptosis suppressor. Oct 2019, Nature, DOI: 10.1038/s41586-019-1707-0.0
People
- Dr. Florencio Porto Freitas works in the research group of Dr. José Pedro Friedmann Angeli at the Rudolf Virchow Center for Experimental Biomedicine of the University of Würzburg.
- Since 2018 Dr. José Pedro Friedmann Angeli is head of a research group at the Rudolf Virchow Center for Experimental Biomedicine of the University of Würzburg.
- Since 2009 Prof. Dr. Markus Sauer has been head of the Department of Biotechnology and Biophysics at Biocenter at the University of Würzburg. Website
- Until Sep 2019 Prof. Almut Schulze was head of a research group at the Chair of Biochemistry and Molecular Biology at Biocenter at the University of Würzburg. Website
The Rudolf Virchow Center
The Rudolf Virchow Center is a central Research Center of the University of Würzburg. Our research groups are studying the structure and function of target proteins in order to understand the causes of diseases.
Contact
Dr. José Pedro Friedmann Angeli (Rudolf Virchow Center)
Tel. +49 931 31-85547, pedro.angeli@virchow.uni-wuerzburg.de
Dr. Daniela Diefenbacher (Press Office, Rudolf Virchow Center)
Tel. +49 931 3188631, daniela.diefenbacher@uni-wuerzburg.de