Focus of Research
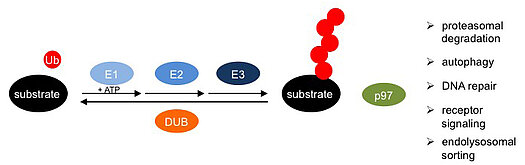
The research program of the GRK 2243 is focused on uncovering molecular mechanisms underlying the cellular functions of key enzymes at all levels of the ubiquitin system: the E1, E2 and E3 enzymes mediating the ubiquitylation of specific target proteins; the de-ubiquitylating enzymes (DUBs) that control, counteract and edit target protein ubiquitylation; and the ATPase p97 (also known as Cdc48 and VCP), an abundant and essential regulator for the turnover of ubiquitylated target proteins. The elucidation of molecular mechanisms and physiological functions of these enzymes will guide the subsequent exploration of their dysfunction in ubiquitin-related diseases and the identification of small-molecule inhibitors as potential lead compounds for future therapeutic approaches.
The research program comprises 15 individual research projects in four core research areas:
A. Mechanism and regulation of ubiquitylation enzymes
The catalytic cascade of E1, E2 and E3 enzymes forms the heart of the ubiquitin system. Research area A is focused on the characterization of the structures, catalytic activities and regulatory mechanisms of these ubiquitylation enzymes.
B. Mechanism and substrate recognition of de-ubiquitylating enzymes
DUBs have now received full recognition both as antagonists and as modulators of ubiquitin chain formation. Research area B is focused on the structural and functional characterization of DUBs that play critical roles in cancer and infectious diseases.
C. Mechanisms of transcriptional control by (de-)ubiquitylating enzymes
Target protein ubiquitylation and de-ubiquitylation play numerous roles in transcription. Research area C focuses on the control of transcription through the ubiquitylation state of two important groups of transcription factors, Myc family members and SREBPs (sterol regulatory element-binding proteins), which are central regulators of cell growth and lipid metabolism, respectively.
D. Mechanism of p97 function in health and disease
The ATPase p97 is essential for various pathways of the ubiquitin system, because it releases ubiquitylated substrates for subsequent proteasomal degradation or non-proteolytic fates. Research area D is focused on the elucidation of the molecular mechanism of p97 function, its control by regulatory cofactors, its mutational impairment in neurodegenerative diseases, and its manipulation by small molecule inhibitors.