P 4: A. Schubert-Unkmeir
Development of multi-cellular in vitro models of the meningeal blood-cerebrospinal fluid barrier for infection studies with Neisseria meningitidis
State of the art
N. meningitidis (the meningococcus) colonizes the nasopharynx mainly as a commensal, being carried asymptomatically by 5–10% of the healthy population in non-endemic times. In rare cases, N. meningitidis can cross the epithelium of the nasopharynx, gain access to the bloodstream and cause severe septicemia and/or meningitis. One of the main factors affecting the pathogenicity of N. meningitidis is the ability to penetrate the vascular endothelial cell layer of the blood-cerebrospinal fluid barrier (B-CSFB) and infect the meninges. Although the role of adhesins and invasins in the virulence of N. meningitidis has been demonstrated, the mechanisms that govern meningococcal penetration of brain endothelial cells (BECs) and subsequent interaction with leptomeningeal cells are still not fully understood.
Previous Work
In the first funding period of the GRK 2157 induced pluripotent stem cells (iPSC)-derived BEC like cells have been implemented as a novel cellular model for N. meningitidis infection in close collaboration with Dr. Antje Appelt-Menzel/PD Dr. M. Metzger (LS TERM Würzburg) and Dr. Brandon Kim (University of Alabama)/Prof. E. Shusta (University of Wisconsin) according to previously described methods [1, 2]. By using gentamicin protection and immunofluorescence assays we confirmed that multiple N. meningitidis wildtype strains and mutants followed similar phenotypes to previously described models (Fig.1). Recruitment of the recently published pilus adhesion receptor CD147 underneath meningococcal microcolonies in iPSC-BECs was demonstrated. N. meningitidis was found to directly disrupt the three tight junction proteins ZO-1, Occludin, and Claudin-5 at late time points of infection, which became frayed and/or discontinuous upon infection. This destruction was preceded by, and might be dependent on, SNAI1 activation (a transcriptional repressor of tight junction proteins). In accordance with tight junction loss, a sharp loss in TEER, and an increase in sodium fluorescein (NaF) permeability could be shown (Fig.1). Notably, bacterial transmigration correlated with junctional disruption, indicating that a paracellular route contributes for bacterial crossing of BECs. In addition, RNA-Seq data analyses of sorted, infected iPSC-BECs were established in collaboration with A. Westermann (HIRI Wuerzburg) providing expression data of N. meningitidis-responsive host genes not previously described thus far to play a role in N. meningitidis infection of BECs [3]. However, while iPSC-BECs formed a reasonable barrier with high TEER values, these cells lack significant cytokine/chemokine secretion after challenge with N. meningitidis [3]. Since the inflammatory response plays a primordial role in the pathogenesis of meningococcal meningitis, we will continue using both, iPSC-derived BEC-like cells as well as hCMEC/D3 cells, that have been established as state of the art in vitro model in recent years for N. meningitidis infection of the B-CSFB [4] .
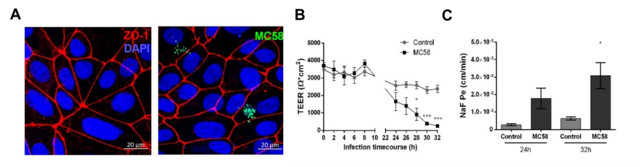
Figure 1: (A) Confocal microscopy images of iPSC-BECs seeded onto ibidi slides infected with GFP-expressing N. meningitidis strain MC58. (B) Polarized iPSC-BEC monolayers seeded onto 0.4 μm pore size 12-well size Corning transwells in the presence of hESFM + 1 % PDS, with or without MC58 challenge at MOI 10 were used for monitoring of TEER values (Ωxcm2) over a timecourse of 32 h, and (C) determination of Na-Fluorescein permeability coefficient (cm/min) at 24 h and 32 h p.i..
Working hypothesis and work plan:
The B-CSFB is composed of brain ECs surrounded by leptomeningeal cells. In the second application period we now use the current model and developed a human B-CSFB in vitro co-culture transfer model. The co-culture model is established consisting of a confluent layer of BECs (iPSC-derived BEC-like cells and hCMEC/D3) on the apical side and a monolayer of leptomeningeal cells on the basolateral side of a microporous membrane for use in mechanistic studies on bacterial transmigration, permeability changes (monitored by NaF transport) and TEER values. Leptomeningeal cells have already been received from our collaboration partner, Prof. M. Christodoulides (University of Southampton) and successfully implemented.
We currently characterize and monitor the quality of the in vitro human B-CSFB co-culture model using transmission electron microscopy (TEM) in collaboration with C. Stigloher (Imaging Core Facility Biozentrum, Würzburg). Sequential events of bacterial adhesion, cellular uptake, localisation and transcytosis are analysed in detail by TEM imaging as well as super-resolution microscopy (SIM and dSTORM/PALM) in collaboration with M. Sauer (Biozentrum, Würzburg).
RNA-Seq data analyses of sorted, infected BECs provided differential regulation of genes involved in hypoxia [3]. Hypoxia is a common feature during inflammation associated with bacterial infection, however the role of hypoxia on N. meningitidis B-CSFB infection has not been elucidated so far. Since activation of hypoxia-inducible factor 1 (HIF-1) transcription factor is the most recognized pathway adopted by hypoxic cells we will determine activation of HIF-1 in N. meningitidis infected BECs and analyse the role of this pathway in maintaining/disturbing B-CSFB function. The contribution of bacterial components (LOS, Pili, NadA) directly involved in HIF-1 activation by the pathogen will be addressed. The different strategies (BECs/leptomeningeal co-cultures, dynamic exposure) are very promising to improve the current in vitro models and will help to reveal useful sub-cellular details to provide evidence for the mechanism of bacterial transmigration in the human B-CSFB model.
References:
1. Appelt-Menzel A, Cubukova A, Gunther K, Edenhofer F, Piontek J, Krause G, et al. Establishment of a Human Blood-Brain Barrier Co-culture Model Mimicking the Neurovascular Unit Using Induced Pluri- and Multipotent Stem Cells. Stem cell reports. 2017;8(4):894-906. 2. Lippmann ES, Azarin SM, Kay JE, Nessler RA, Wilson HK, Al-Ahmad A, et al. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nature biotechnology. 2012;30(8):783-91. 3. Martins Gomes SF, Westermann AJ, Sauerwein T, Hertlein T, Förstner KU, Ohlsen K, Metzger M, Shusta EV, Kim BJ, Appelt-Menzel A, Schubert-Unkmeir A. Induced Pluripotent Stem Cell-Derived Brain Endothelial Cells as a Cellular Model to Study Neisseria meningitidis Infection. Frontiers in Microbiology. 2019;10:1181. 4. Bernard SC, Simpson N, Join-Lambert O, Federici C, Laran-Chich MP, Maissa N, et al. Pathogenic Neisseria meningitidis utilizes CD147 for vascular colonization. Nat Med. 2014;20(7):725-31. 5. Mairey E, Genovesio A, Donnadieu E, Bernard C, Jaubert F, Pinard E, et al. Cerebral microcirculation shear stress levels determine Neisseria meningitidis attachment sites along the blood-brain barrier. J Exp Med. 2006;203(8):1939-50.






