Switch for DNA repair tool discovered
04/03/2020Scientists from the University of Würzburg and the University of Strasbourg identified a new important molecular region in an essential human DNA repair complex, consisting of the proteins XPD and MAT1. This complex forms a central unit in the nucleotide excision DNA repair mechanism (NER) and thus protects our genetic information. The findings were published in the journal Nature Communications and could provide new starting points for cancer therapy.
The human genome is constantly exposed to harmful factors, such as the UV light of the sun. Cancer or premature aging can be the result, if these damages in the DNA are not repaired just in time. Over the course of time, our organism has developed efficient repair mechanisms that can counteract a wide variety of damage in the genetic material. The NER is one of these mechanisms. In order to coordinate these complex biological processes, it is very important that all components involved are strictly regulated and coordinated. The XPD protein plays a key role in DNA repair.
Important functional region in DNA repair-complex identified
To gain a better understanding of the interaction of the proteins involved, the research groups of Prof. Dr. Caroline Kisker from the Rudolf Virchow Center for Experimental Biomedicine at the Julius Maximilian University (JMU) Würzburg and Prof. Dr. Jean Marc Egly from the Institut de Génétique et de Biologie Moléculaire et Cellulaire at the University of Strasbourg (France) analyzed their molecular structure.
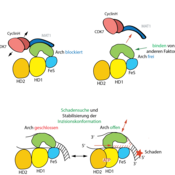
In a combinatorial approach, the two research groups have been able to identify a new functional region in the XPD protein, which is mechanistically essential for XPD activity as well as for interaction with other proteins. "Our structural biology analyses have made it possible to decipher the mechanism of XPD regulation through MAT1, which in turn allows important conclusions to be drawn about the XPD function," reports Kisker.
MAT1 is the most important interaction partner
A central unit of NER is the protein complex TFIIH, which consists of ten subunits. The XPD protein plays a key role in TFIIH, both in DNA repair and in transcription, i.e. the normal reading of DNA. However, the protein must only be active as an enzyme during its repair function. The scientists have now been able to show how the XPD is switched on and off in different situations: The protein MAT1 is extremely important as an interaction partner for its regulation.
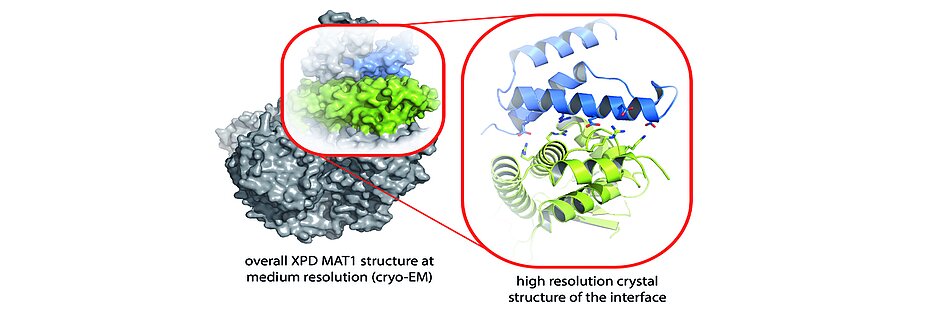
The research groups succeeded in identifying the molecular three-dimensional structure (crystal structure) of the XPD-Arch domain and its interaction partner MAT1. “This enabled us to analyze functionally important areas within the interaction area in the XPD protein that are essential for a functional NER,” explains Dr. Jochen Kuper. On one hand, these areas are directly linked to the activity of the protein and, on the other hand, they regulate subsequent processes by recruiting and positioning important "downstream" NER components. If MAT1 is bound to XPD, these areas are covered and XPD is inactive.
XPD in cancer therapy
In the future the major focus will be on the function of NER, as this essential mechanism also plays a role in the treatment of cancer. Many cancer chemotherapeutic agents attack the DNA of cancer cells, cause significant damage to their genome and thus lead to the death of the cancer cells. However, an effective DNA repair mechanism in cancer cells can prevent this and thus severely weaken the effect of the therapeutic agent. DNA repair inhibitors might open up an attractive method in cancer treatment.
"We can already see that NER can provide important target structures for cancer therapy. The XPD protein in particular plays a key role due to its central importance," emphasizes Kisker. "We are currently working intensively on analyzing other central components of TFIIH in order to clarify their structure-function relationship. In addition, we have initiated a campaign to identify active substances against the XPD protein and to test them for their therapeutic usefulness," add Kisker and Kuper.
Publication
Stefan Peissert, Florian Sauer, Daniel B. Grabarczyk, Cathy Braun, Gudrun Sander, Arnaud Poterszman, Jean-Marc Egly, Jochen Kuper and Caroline Kisker: In TFIIH the Arch domain of XPD is mechanistically essential for transcription and DNA repair. Nature Communications (April 2020), doi 10.1038/s41467-020-15241-9
Persons
Dr. Jochen Kuper (Postdoc) is a researcher in Prof. Dr. Caroline Kisker's group at the Rudolf Virchow Center for Experimental Biomedicine at the University of Würzburg.
Prof. Dr. Caroline Kisker is, among other things, head of the Chair of Structural Biology and Dean of the Graduate School of Life Sciences at the University of Würzburg. Since April 2016, she has been part of the dual leadership of the Rudolf Virchow Center for Experimental Biomedicine at the University of Würzburg. More information: https://www.uni-wuerzburg.de/rvz/lehrstuehle/lehrstuhl-fuer-strukturbiologie/about/
Kontakt
Prof. Dr. Caroline Kisker (Chair of Structural Biology, Rudolf Virchow Center )
Tel. +49 (0)931 31 80381, caroline.kisker@virchow.uni-wuerzburg.de
Dr. Daniela Diefenbacher (Press Office, Rudolf Virchow Center )
Tel. +49 (0)931 31 88631, daniela.diefenbacher@uni-wuerzburg.de