Faster Determination of Protein Affinities
01/29/2021Protein interactions can now be analysed more effectively: A phenomenon called Temperature Related Intensity Change (TRIC) makes it possible to achieve high binding signal intensity even with lowest amounts of sample. Our Maric research group established the method, which can be used to study protein complexes in highly regulated networks such as DNA transcription.
Limitations of currently available methods
Protein-protein interactions (PPIs) play critical roles in virtually all physiological processes. To explore novel therapeutic approaches it is essential to quantify and understand these interactions. Various approaches for the quantification of protein interactions have been developed; their application however, is typically hampered by low throughput and elaborate sample preparation.
Combination of fast synthesis and fast binding evaluation
Scientists from our research group of Dr. Hans Michael Maric together with experts from Nanotemper Technologies in Munich have now demonstrated how to overcome the aforementioned limitations.
“We have established an accessible setup that combines high-throughput synthesis with binding evaluation,” says Maric. The new setup allows for the precise determination of binding affinities of unmodified ligands in solution. “By providing molecular level insights in solution, the new setup complements our array based approach at a level of detail that we could only achieve using laborious low-throughput techniques,” explains Maric.
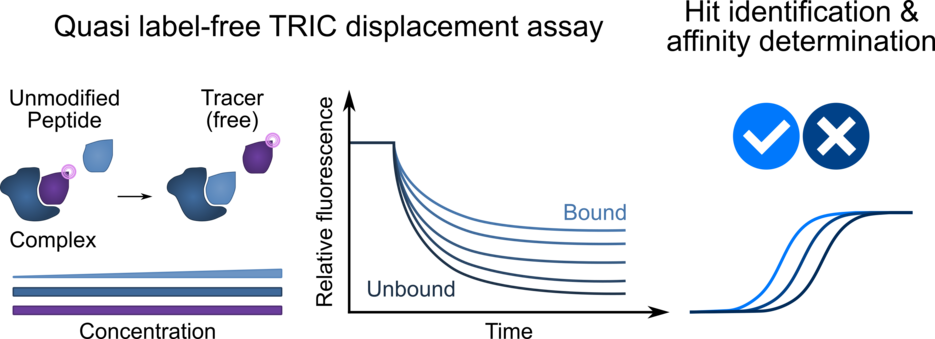
Size independent measurements using TRIC
In contrast to other widely used high-throughput quantification approaches, the introduced TRIC measurement is largely independent of ligand size. “Using a displacement setup, this approach not only enable the measurement of unmodified ligands, but also boosts the signal compared to other measurement methods,” explains Clemens Schulte.
Automatization and commercialization
Amit Jean Gupta at Nanotemper Technologies is responsible for the commercialization of the “Dianthus” which allows for widely automated TRIC measurements. “Conventional Microscale Thermophoresis (MST) setups require tracking the molecular motion along a temperature gradient in a vessel that prevents turbulent flow. Relying solely on the TRIC-principle allowed us to reduce volumes and achieve full compatibility with microtiter plates, thus automation and enhanced throughput, while maintaining the advantages of conventional MST measurements. The work of the Maric team establishes TRIC measurements as an accessible, high-throughput PPI quantification approach and provides a key reference for future applications,” says Amit Gupta.
Further work is required
Within the current setup, thousands of peptides are first synthesized by an automated system and then directly applied to the high-throughput binding evaluation. Here, additional universal peptide purification steps could greatly enhance the robustness, sensitivity and predictive value of the method, especially for peptides exceeding 20 amino acids. "The enhanced miniaturization and automatization further improved our ability to decipher complex protein-protein interactions networks. When applied iteratively, our technology enables the development of highly specific binders. We will use our setup to study and target specific protein complexes in complex and highly regulated networks such as DNA transcription and the function of the neuronal synapse,” concludes Maric.
Publication
Clemens Schulte, Vladimir Khayenko, Noah Frieder Nordblom, Franziska Tippel, Violetta Peck, Amit Jean Gupta, Hans Michael Maric,High-throughput Determination of Protein Affinities using Unmodified Peptide Libraries in Nanomolar Scale. iScience, January 2021, DOI: 10.1016/j.isci.2020.101898
Contact
Dr. Hans Michael Maric (Rudolf Virchow Center, University of Würzburg)
Tel.: +49 931 31-85371, Hans.Maric@uni-wuerzburg.de
Clemens Schulte (Rudolf Virchow Center, University of Würzburg)
Tel.: +49 931 31-89734, Clemens.Schulte@uni-wuerzburg.de