Guardian of the genome: Structure of key enzyme decoded
07/05/2017Scientists from the University of Würzburg solved the structure of the human protein RecQ4 and gained insights into its unusual functional mechanisms, which could help towards the development of new therapeutic strategies against certain tumors.
Living organisms aim to preserve their genomic integrity to maintain cellular functions and to pass on the correct genetic information to following generations. An important role in preserving genetic information is performed by RecQ helicases, a group of enzymes that is involved in central DNA-based processes, including DNA replication, recombination and repair. RecQ helicases are highly conserved among all living organisms, from bacteria to humans.
In humans, malfunction of these important enzymes causes serious chromosomal damages, resulting in fatal diseases. The development of novel therapies to treat such diseases relies on the detailed knowledge of how these RecQ helicases operate at the molecular level.
RecQ4 is involved in cancer development
Scientists of the Rudolf Virchow Center for Experimental Biomedicine at the University of Würzburg investigated a particular member of this central enzyme family, called RecQ4. It is known that an impairment of RecQ4 function promotes the development of different types of cancer and provokes symptoms of premature aging. Moreover, mutations in the RecQ4 helicase are linked to systemic diseases like the Rothmund-Thomson-Syndrome and RAPADILINO-Syndrome, which are characterized by skeletal abnormalities and growth retardation.
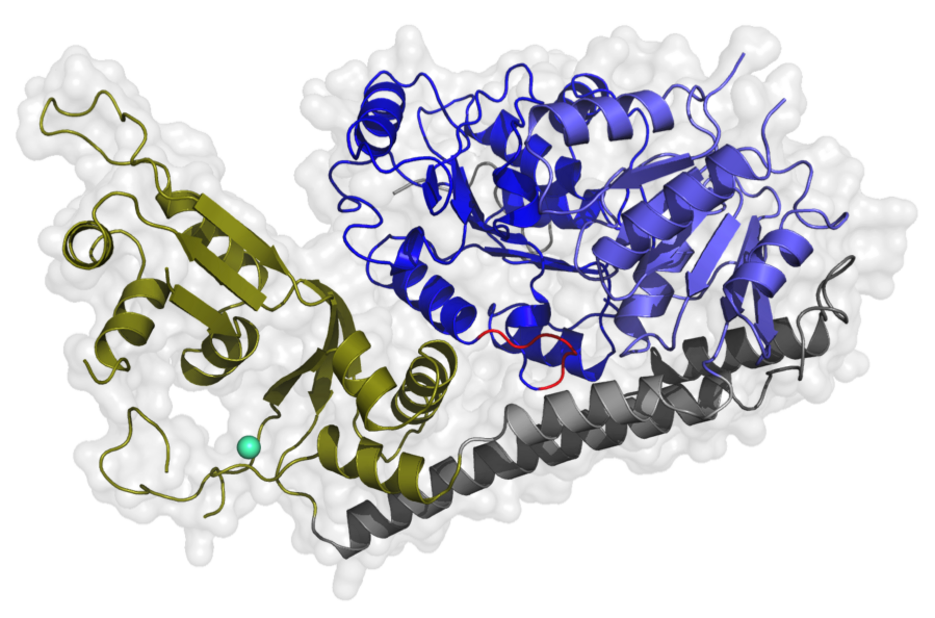
Researchers from the group of Prof. Caroline Kisker now successfully determined the crystal structure of the human RecQ4 helicase and identified unique domains in this protein, which are not shared with any other RecQ member. While the helicase core comprises the conserved structural fold of a molecular power station that provides the energy to unwind the double-stranded DNA, further functional domains that are characteristic for the enzyme family are missing. Instead, the scientists identified a novel protein domain that is potentially responsible for specific RecQ4 functions.
An uncommon mechanism
Due to the special structural properties of RecQ4, the scientists assume an uncommon helicase mechanism. "Other human RecQ helicases use a molecular wedge element to seperate the double-stranded DNA", explains Prof. Kisker. "In RecQ4 we could not identify such a structure so far. That leads to the question, how the enzyme performs this strand separation mechanism?" Potentially, RecQ4 could strongly bend the DNA in order to weaken the paired bases and thereby separate the DNA strands - a mechanism known from bacterial RecQ helicases. An exceptional role for RecQ4 is also assumed based on its special cellular distribution: it is the only RecQ helicase, which localizes to mitochondria in addition to the cell nucleus and the cytoplasm. Thus, the enzyme might also be involved in replication and preservation of mitochondrial DNA.
Development of therapeutic approaches
Recently published in Nature Communications, the results on the RecQ4 helicase provide novel insights into the architecture and function of this important protein. Moreover, the structure of RecQ4 represents a valuable model to understand and investigate the development of RecQ4-associated diseases. Finally, due to the unique structure and the prominently elevated expression levels in cells of different cancer types, RecQ4 could resemble a promising target to develop novel therapeutic approaches in the fight against cancer.
Publication:
Kaiser S., Sauer F., Kisker C. (2017) The structural and functional characterization of human RecQ4 reveals insights into its helicase mechanism. Nature Communications 8, 15907
Websites:
AG Kisker
Kisker Lab
Kontakt:
Prof. Dr. Caroline Kisker (Structural Biology, Rudolf Virchow Center)
Tel. +49 (0)931 31 - 80381, caroline.kisker@virchow.uni-wuerzburg.de
Created By:
Dr. Frank Sommerlandt (Public Science Center, Rudolf Virchow Center)
Tel. +49 (0)931 31 - 88449, frank.sommerlandt@uni-wuerzburg.de