P13 - Physical principles of parasite-host interactions in Giardia muris infection
Dissecting physical principles of parasite-host interactions in Giardia muris infection
Giardia are parasitic protists whose trophozoite stage infects the upper intestinal tract of humans and many other vertebrates. This habitat is rich in nutrients, but the parasite must avoid being excreted via peristalsis. In addition, close contact of the parasite with the rapidly renewing epithelial cells necessitates regular changes of location, and the proximity to the underlying immune cells makes the trophozoites an easy target for host defense. Finally, the uneven terrain is covered by viscoelastic mucus studded with defense substances.
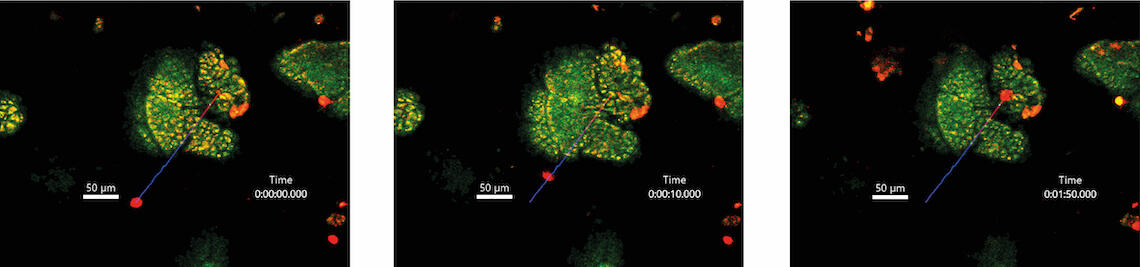
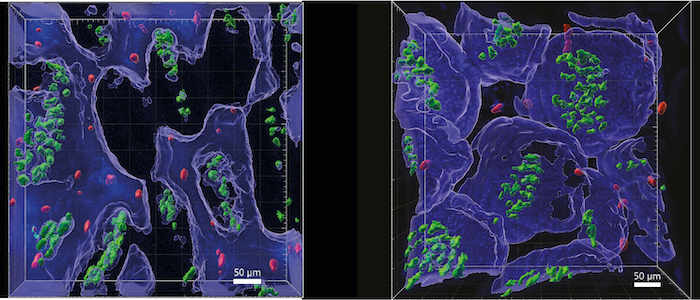
To overcome these challenges, Giardia have evolved several impressive adaptations. An adhesive disc allows trophozoites to anchor within fractions of a second, and renewal of this organelle during cell division takes only minutes. In addition, Giardia possess four pairs of flagella that allow penetration of and locomotion in mucus. This illustrates that Giardia use physical principles to adapt to their host habitat. Our project therefore addresses two overarching questions: 1) How flexible are Giardia in their lifestyle in response to force dynamics in the host organism? 2) Do host cells sense and respond to mechanical forces exerted by Giardia? In examining the physical aspects of a natural parasite-host interaction, we will first focus on the parasite. Using intravital microscopy, we will quantitatively analyse the motility of G. muris in the mouse intestine, its natural environment. Mechanical forces exerted during attachment of G. muris to the intestinal epithelium will be determined via atomic force microscopy using intestinal organoids. To address the bilateral nature of the parasite-host relationship, we will further dissect the effects of G. muris attachment on host cells. Here, we will focus on how the process is perceived by different cell types and how the resulting forces are transmitted to the intestinal tissues. Finally, we will use genetic and pharmacological interventions to investigate whether mechanosensation of intestinal barrier cells influences control of infection.
Our project offers the opportunity to elaborate a variety of synergies within DFG Priority Program 2332. First, the project is focused on a parasite that shares physical principles of its own locomotion and adhesion, as well as challenges posed by the colonised environment, with other organisms being worked on in this Priority Program. Second, we are using and sharing specialized microscopy techniques and novel functional analysis systems to study host responses to mechanical forces exerted by parasites