P8 - Structural determinants and function of chirality in the motion of malaria parasites
Chirality in Plasmodium
Chirality is present in biological systems from the molecular to the cellular to the organismal level, but usually it is difficult to connect it across scales. Most biomolecules are chiral and this can be used by biological systems to break symmetries and to develop a body plans with clearly defined axes and chiral elements. For example, the left/right turns of snailshells have been traced back to the action of formins polymerising actin filaments. The fact that the heart is usually on our left side (except for the rare cases of situs inversus) has been traced back to the beating of the cilia in the nodal region, generating unidirectional hydrodynamic flows.
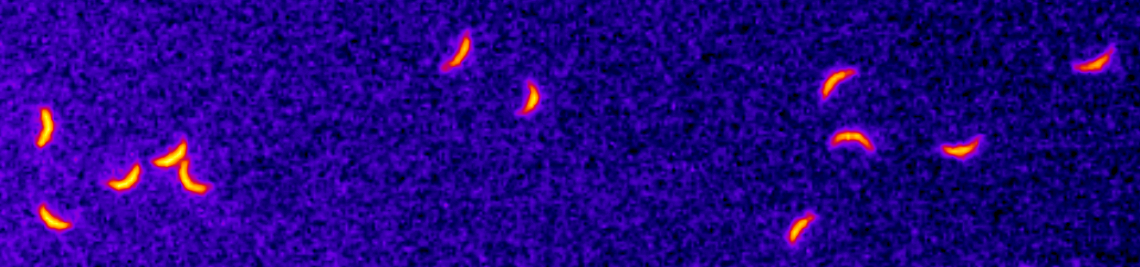
Apicomplexans such as Plasmodium (causative agent of malaria) or Toxoplasma (causative agent of toxoplasmosis) are unicellular parasites which show chiral signatures in their cell migration patterns: they tend to move in circles of a specific orientation on flat substrates (counterclockwise in the usual setup), and follow helical paths when in hydrogels or tissue. This chirality might help to overcome mechanical barriers in the challenging environment of their host, e.g. when Plasmodium sporozoites have to move through the skin to find blood vessels that transport them to the liver, where the next stage of a malaria infection starts. It is however not understood how chirality in cell migration emerges from the underlying biomolecular organisation, and what exact function it plays during transmission and infection.
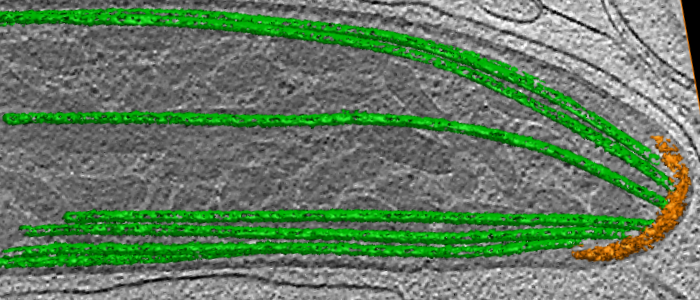
In this project, we will study two life cycle stages of Plasmodium (ookinetes and sporozoites) that exhibit chirality during their migration. We also will compare the results to Toxoplasma as a similar but different reference system. As shown in the electron microscopy image of the Plasmodium sporozoite, such apicomplexans have a characteristic subpellicular microtubule basket (green) that fans out from the polar ring (orange) at the front of the cell. We will investigate if these microtubules might underlie the cellular chirality observed during migration. Another potential source for this chirality might be the actomyosin system responsible for the gliding motility of Apicomplexa.
On the biological side, we will use genetic and imaging methods to attack this question. On the physics side, we will use traction force microscopy and quantitative image analysis to characterise the chiral motion of malaria parasites. We will then link these data to simple models for self-propelled active particles of non-spherical shape, examining what happens when translation and rotation are coupled. Finally, we will investigate the micromechanics of malaria parasites using the finite element method.